Oncology drug development continues to increase in its complexity. Large contract research organizations (CROs) are well-suited for less complex therapeutic areas but weren’t established to operate in the current and evolving oncology paradigm.
As an example, since 2023, the Project Optimus initiative from the U.S. Food and Drug Administration (FDA) has spurred larger, more complex early phase study designs that require expertise and flexibility to adapt to emerging dose optimization data.
As next-generation precision and immuno-oncology therapies proliferate, oncology patients’ drug toxicity profiles (safety) and clinical responses (efficacy) become more complex to understand and manage. Patient access remains limited, while we continue to look for more and more targeted populations, requiring a global footprint as well as sites that can truly drive recruitment goals. And on top of that, oncology studies often involve more logistical challenges around investigational medicinal product (IMP) management, such as complex packaging, shipping and storage requirements for immunotherapies, just-in-time dosing or special handling requirements for radiopharmaceuticals or cell therapy.
A specialty oncology CRO, such as Catalyst Oncology, has the expertise to understand and proactively manage the growing complexity of oncology drug development. A global specialist, Catalyst Oncology can help take you from a clinical development plan to first-in-human (FIH) all the way through to approval and commercialization. Specialty oncology CROs, in comparison to large generalist CROs, serve the best interests of biotech sponsors.

Deep oncology expertise throughout each function
Many people working in oncology do so for personal reasons. As a specialty CRO, our exclusivity to oncology enables the recruitment and retention of top talent who are passionate about finding new cancer therapies. We infuse oncology expertise throughout every function, guaranteeing you an oncology-seasoned study team that understands the latest trial complexities and risks.
Although many large CROs may claim to have oncology divisions or specialty teams, often these are just one function: project management. Biotech companies need to ask if the rest of the CRO team such as clinical research associates (CRAs), data managers, statisticians or safety specialists are exclusively oncology or resourced from a shared pool across multiple therapeutic areas. Once you do, you will quickly see the risk of getting the B team and the associated time consumed teaching the large CRO team how to interact with an oncologist, how to calculate a partial response, or how to properly watch for and capture adverse events of special interest (AESIs).

Proud to be exclusively biotech
While many large CROs have biotech-centric divisions and many mid-sized CROs claim to be built for biotech, once you ask if there are separate standard operating procedures (SOPs) or systems for biotechs, you will quickly realize the difference.
Scratch one layer deeper and ask a large CRO about the percentage of biotech customers. Once you do, you’ll learn a business designed entirely around biotech sponsors enables:
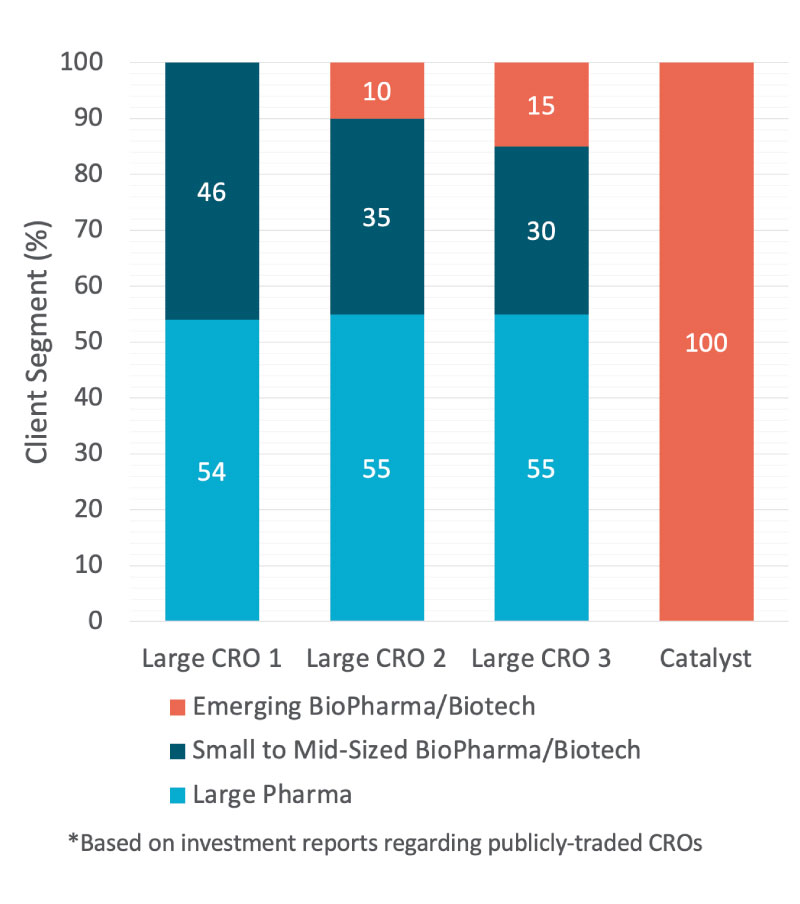
An understanding of corporate milestones
A sense of urgency
Thoughtful management of your budget (a partner that won’t nickel and dime you)
Flexibility to adapt to emerging data in real time
Transparent communication
Instant access to senior leadership
A biotech-exclusive CRO ensures you have the team and the attention you deserve
Nimble processes with industry-leading technologies
Large CROs still operate with a one-size fits all approach that creates lengthy, cumbersome processes too rigid and inflexible for the shifting oncology development paradigm.
Catalyst Oncology is regularly asked to rescue studies from big, general CROs unable to move quicky enough or to work while the protocol is being finalized. Or an IND submission to hit a critical first-patient milestone. We’ve rescued large Phase III studies where the non-specialty CRO is unresponsive, uses out-of-date technology, or has been significantly behind with locking patients who have been off a study for months, which prevents you from getting an early look at your data.
Whether complex trial logistics or ongoing protocol changes, an oncology CRO is accustomed to the nuances and challenges of cancer trial designs. A specialty CRO can proactively adapt and quickly respond to changing study needs. Leveraging an oncology CRO built from the ground up with industry-leading technologies with access to big data on par with generalist CRO offerings assures your asset and clinical trial will come out ahead of where it would be, not behind plan and in need of a rescue.
Specialty oncology CRO with a global footprint
With less than 10% of oncology patients participating in clinical trials, oncology study recruitment can be slow. Combine that with targeted sub-indication treatments and the trend brought upon by the FDA’s Project Optimus for larger sample sizes to better explore dose optimization and oncology studies as early as first in human (FIH) now require global patient access.
Catalyst Oncology, a specialty oncology CRO with more than 1,000 staff and operations across North America, Europe, and the Asia-Pacific region, easily supports multinational FIH through to registrational Phase III trials, giving you access to patient populations to help with timely enrollment.

Experts in oncology data review and interpretation
Because oncology trial designs are complex with large amounts of data, it is critical to continually monitor study data in real-time, looking for early safety and efficacy signals. Large CROs perform basic data review, such as standard electronic data capture (EDC) queries or monthly aggregate checks but are not sophisticated enough to monitor data as critically or as often as oncology studies require.
A biotech-only and oncology-only CRO leverages a data-centric approach using clinical scientists, who are experts in data review and interpretation with a study’s endpoints and a biotech sponsor’s goals in mind. The specialty oncology CRO uses a custom-built clinical analytics tool that specifically helps visualize oncology safety and efficacy datapoints in near-real time. This expertise enables quick responses in early phase development where positive safety or efficacy signals can trigger a breakthrough development pathway or in late phase development where interim looks, quality, and submission-ready data are key.
Quality site relationships
A large generalist CRO’s site networks are based on volume, require redundant startup processes, and provide little value to the sites themselves. A specialty CRO approach forges deep relationships with a smaller number of quality sites, fostering a true CRO-site partnership. With an oncology CRO, sites receive value from their contributions, appreciate personal contacts, and streamlined startup processes, along with the first right of refusal to cutting-edge therapies. These relationships—marked by mutual trust and respect—better enable a specialist CRO to meet a sponsor’s study enrollment and retention goals.
While many companies benefit from the services of a large generalist CRO, biotechs may not be in that category. A specialty oncology CRO, such as Catalyst Oncology, serves the needs of innovative biotechs because they are our sole focus. We have a depth and breadth of knowledge, experience, and a passion that permeates our global teams. We look forward to speaking with you and learning how we can support you and your study goals, from early planning to full-service clinical trial implementation.