“Today, overall cancer clinical trial enrollment in the United States is 8% (6.3%‐7.0% at community centers and 14.0%‐15.9% at academic centers), and Black, Indigenous, and People of Color patients represent only approximately 15% of that low overall participation. With diverse ethnic and racial groups composing nearly 40% of the US population, this staggering mismatch of racial and ethnic representation in cancer clinical trials must be addressed.” 1
FDA guidance on diversity in clinical research
Historically, the pharmaceutical industry has been challenged by underrepresentation of diverse populations in clinical trials. The U.S. Food and Drug Administration (FDA) issued “Enhancing the Diversity of Clinical Trial Populations—Eligibility Criteria, Enrollment Practices, and Trial Designs Guidance for Industry” in 2020 to increase awareness and enrollment of minority populations in clinical trials. Trial data should “consider using the real-world data,” 2 therefore proactive diversity, equity, and inclusion (DEI) planning and intervention should start in early phase drug development.
The FDA suggests that clinical trials reflect subject populations in both demographic, such as age and ethnicity, and non-demographic features, which can include comorbidities and those with conditions having a narrow prevalence. 3
Catalyst values influence diversity commitment for your trial
Catalyst Oncology has a robust set of values that enhance our approach toward DEI in cancer trials. Our commitment to our clients is to help bring their life-altering therapies to patients who need them. We support and collaborate with sponsors and sites to develop outreach initiatives that will increase enrollment as well as address population disparities.
Because we understand that without enrolling underrepresented patients, a therapy’s efficacy may be misrepresented in certain populations, we are committed to increasing diversity in clinical trials. We listen to a sponsor or site’s needs and partner to determine a solution, doing what is needed for each individual trial.
Catalyst Oncology’s value of continuous learning provides the backdrop to our understanding and development of flexible approaches toward recruiting diverse populations. We are willing to do what is needed to ensure results reflect multiple populations represented by demographic and non-demographic measurements. As part of the clinical development plan, a DEI strategy can be developed, deployed, and augmented to address any disparities.
Catalyst Oncology recommends routine monitoring and reporting of diversity metrics throughout the study to heighten team awareness and allow for readjustment of tactics as required.
Catalyst values in action
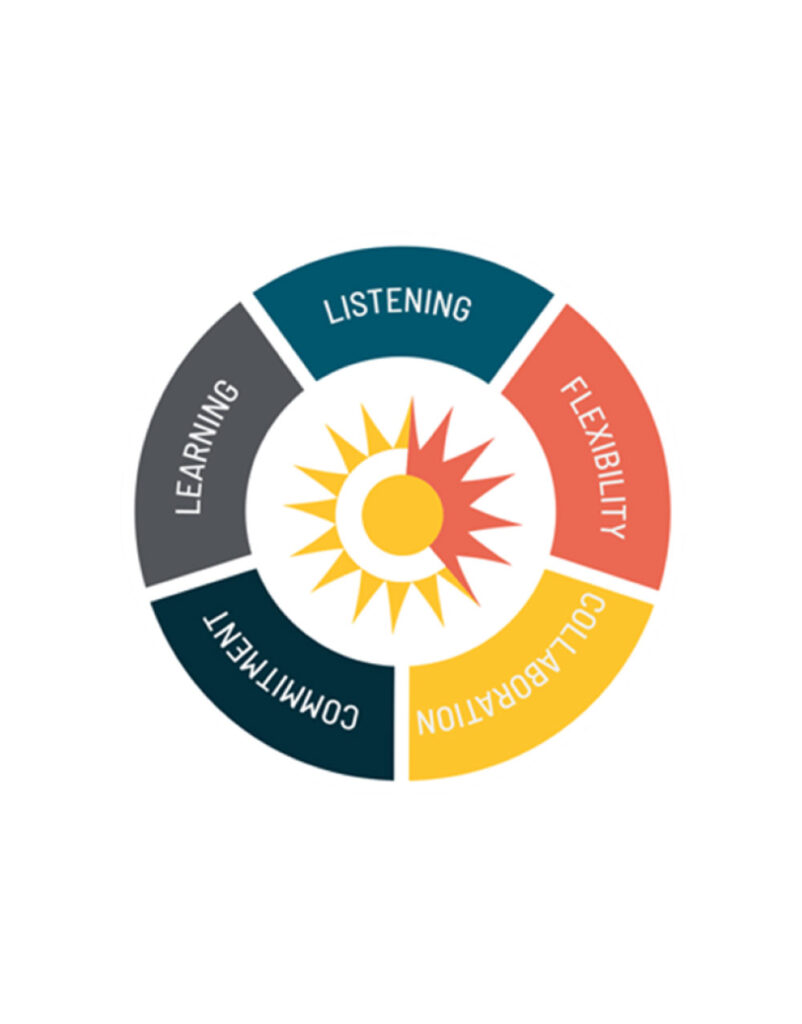
Learning
Aware and informed of initiatives and solutions
Listening
Attentive to the barriers and challenges
Flexibility
Pivot for the unexpected and provide workable solutions
Collaboration
Cooperate and support local doctors to increase their willingness to offer clinical trial options, educate patients, improve trust, and perceptions
Commitment
Provide thoughtful, meaningful approaches to expand clinical trial awareness and participation to do better for diversity
As diversity in clinical trials continues to grow in its importance, advocating for and building trust in diverse population groups will strengthen clinical trials. To help address diversity concerns, partner with Catalyst Oncology, a specialty contract research organization with a commitment to inspiring people to design and deliver better clinical trials—for all.
1 Kahn JM, Gray DM 2nd, Oliveri JM, Washington CM, DeGraffinreid CR, Paskett ED. Strategies to improve diversity, equity, and inclusion in clinical trials. Cancer. 2022 Jan 15;128(2):216-221. doi: 10.1002/cncr.33905. Epub 2021 Sep 8. PMID: 34495551; PMCID: PMC9293140.
2 FDA. Enhancing the Diversity of Clinical Trial Populations—Eligibility Criteria, Enrollment Practices, and Trial Designs Guidance for Industry. 2020. https://www.fda.gov/media/127712/download.
3 Ibid.