Oncology clinical research is complex, demanding, and continually evolving. Bringing effective radiopharmaceutical therapies from proof-of-concept and then to market as fast as possible is our focus because every second counts for patients with cancer and their families.
The promise of radiopharmaceutical research
The need for new, innovative therapies for cancer has never been greater. There were an estimated 20 million new cancer cases and 9.7 million deaths in 2022, and the incidence of cancer is expected to continue rising.
Radiopharmaceuticals are a relatively new, evolving class of cancer therapy proving highly effective at diagnosing and treating cancer. In contrast to traditional radiation therapy, which uses external beams or brachytherapy that often affects both cancerous and healthy tissue, radiopharmaceuticals target and kill cancer cells by delivering precise, DNA-damaging radiation therapy directly to tumors.
Exclusively biotech-focused
Built to work exclusively with biotechs, our flexible processes and approach allow us to listen first, align goals, and execute with an eye toward rapid shifts when protocols amend or Breakthrough designations move a product straight from Phase I to registration.
Seasoned oncology experts
With an average of 9+ years of oncology experience for key roles (PM, CTL, DM, Clinical Science), our oncology specialty guarantees you a seasoned team across all functions.
Additional differentiators
Active next-gen oncology experience
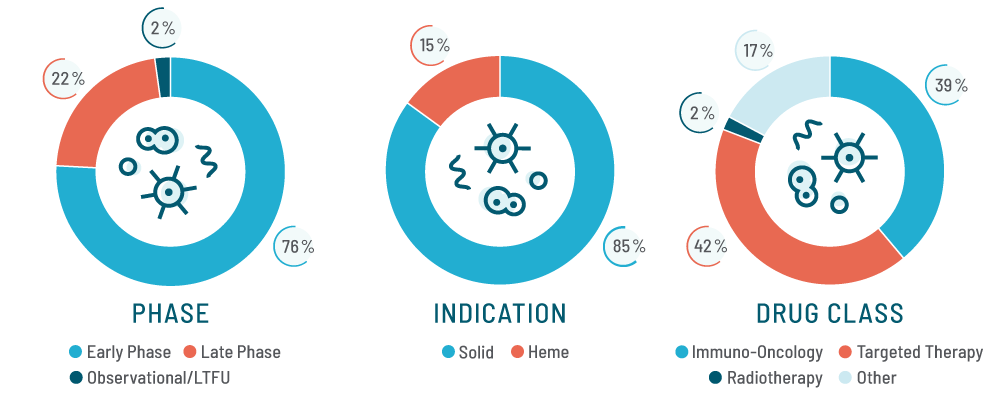
Working both locally and globally across Phases I through III in a range of solid tumor and hematologic indications, we understand the nuances of complex study designs, novel endpoints, and cutting-edge technologies.