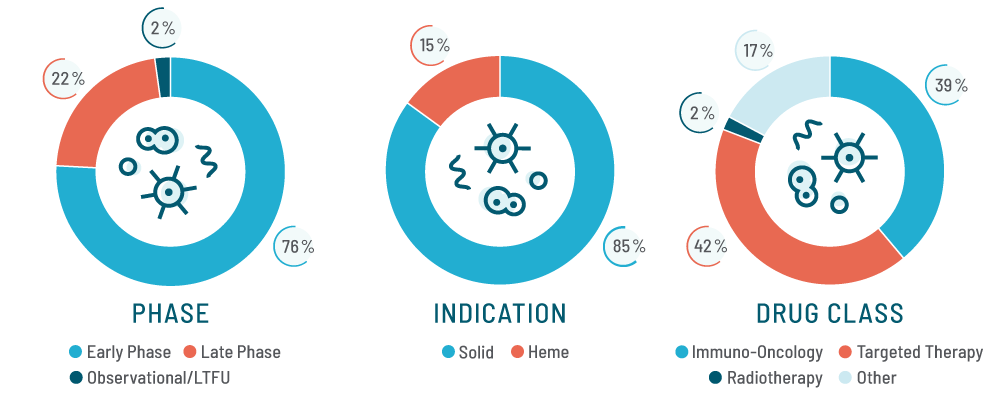
Early-phase oncology trials are complex, demanding precision, rapid execution, and specialized expertise. Yet, too often, the operational burden falls on clinical sites―already overworked and stretched thin. Within large, volume-driven CRO networks, this creates a system that prioritizes scale over quality, offering minimal site support, delaying timelines, and ultimately compromising trial outcomes.
Purpose-built site network designed for biotech
Catalyst Oncology takes a different approach―offering a purpose-built, multiregional site network designed specifically for early-phase oncology trials. Built on rigorously selected sites with proven oncology expertise, this network thrives on trust-based partnerships that streamline operations and eliminate inefficiencies. By easing the burden on sites, we foster a highly motivated network that consistently meets enrollment targets―accelerating timelines, enhancing data integrity, and delivering meaningful clinical results.

Global, relationship-driven site solutions that deliver
Access to global footprint
- ~20 specialized sites located across the US, Spain, UK and Australia
- 50+ active trials at network sites
Collaborative, relationship-driven approach
- Dedicated relationship managers
- First right of refusal for novel protocols with slot and activation commitments
- Early access to next-gen oncology therapies
- Ongoing, consistent support
Operational efficiency and streamlined start-up
- Master templates, core feasibility, waived SEVs and site checklists
- Real-time performance feedback and reliable forecasts
- Expedited launch, elimination of inefficiencies, reduced site fatigue
Tailored, data-driven solutions
- Assessments of site capabilities and financial needs
- Customized tech and resourcing strategies
Exclusively biotech-focused
Built to work exclusively with biotechs, our flexible processes and approach allow us to listen first, align goals, and execute with an eye toward rapid shifts when a protocol is amended or Breakthrough designation moves a product straight from Phase I to registration.
Seasoned oncology experts
With an average of 9+ years of oncology experience for key roles (PM, CTL, DM, Bios, Clinical Science), our oncology specialty guarantees you a seasoned team across all functions.
A transparent people-first culture with industry leading retention
We are responsive and attentive to the needs of both our customers and staff. Our industry-leading employee and project team retention guarantees program continuity, increased efficiency, and happy investigative sites.
Additional differentiators
Catalyst Oncology offers a flexible data-driven approach with an experienced network of sites across a global footprint.

Active next-gen oncology experience
Working both locally and globally across Phases I through III in a range of solid tumor and hematologic indications, we understand the nuances of complex study designs, novel endpoints, and cutting-edge technologies.